Background:
Bendamustine +/- anti-CD20 antibody is a highly effective regimen for iNHL. Though initially favoured for its toxicity profile, subsequent analyses demonstrate profound and prolonged lymphopenia and the landmark phase III GALLIUM study showed a grade 3-5 infection rate of 20-26% in the bendamustine arms (Hiddemann JCO 2018). The relationship between severity and duration of lymphopenia and infection, and the role of antimicrobial prophylaxis (ppx), are not fully characterised. We performed a multicentre, retrospective analysis of bendamustine-treated iNHL patients (pts) to define the type and onset of infections, identify concomitant risk factors and evaluate the role of ppx.
Methods:
iNHL pts aged ≥18 yrs, treated with bendamustine +/- anti-CD20 in 1st-3rd line from 2011-2019, were identified from 9 Australian centres. HIV, prior transplant and long-term immunosuppression were excluded. Demographics, treatment, lymphocyte counts, infections and ppx were collected from baseline to 24 months post end of bendamustine treatment (EOT) or subsequent lymphoma therapy. Association between potential risk factors and infection was evaluated by logistic regression (odds ratio, OR) and negative binomial regression (incidence rate ratio, IRR) with Stata 16.1.
Results:
302 pts were eligible. Baseline and treatment characteristics are summarised in Table 1.
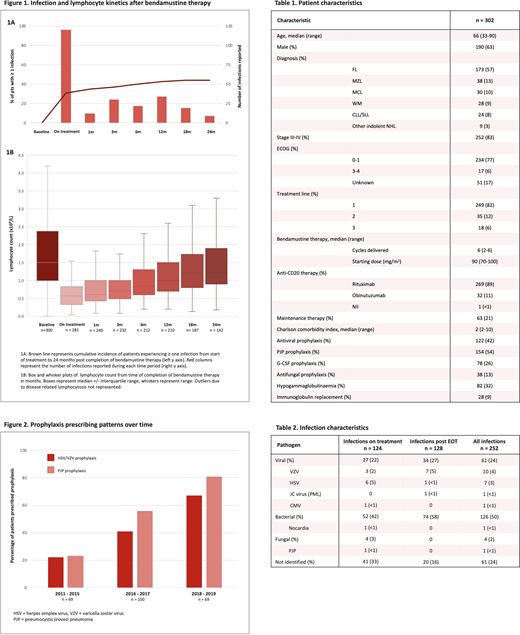
252 infection episodes occurred across 134 pts (44%), equally divided between during therapy and after EOT (Figure 1A, Table 2). Infections on treatment occurred in 30% of pts (n=92) with 18% hospitalised (n = 54; n = 20 with febrile neutropenia (FN)) and dose delay /modification/ discontinuation in 11%. Late infections post EOT occurred in 23% of pts (n=70) with 11% hospitalised (n = 32; n = 12 with FN); infection post EOT was more common in pts on maintenance anti-CD20 (infection rate 49% v 16%, OR 5.1 p<0.001).
Opportunistic infections (OI) occurred in 21 pts: VZV (n=9; 4 on treatment, 5 post EOT, 1 on ppx); HSV (n=5, all on treatment without ppx); PJP (n=1, on treatment without ppx); nocardiosis (n=1, on treatment); other fungal infections (n=3, all on treatment); PML (n=1, 1-yr post EOT); CMV (n=1, at EOT).
Lymphopenia was near universal and prolonged; 98% of pts became lymphopenic (53% grade 3, 9% grade 4) with a median nadir of 0.4x109/L (range 0-2.3). Median time to recovery (>1x109/L) was 10 months post EOT; 39% of pts remained lymphopenic (4% grade 3/4) at 2 yrs (Figure 1B). However, neither lymphopenia nadir nor duration correlated with infection post EOT (OR 0.53 p=0.26 and 0.97 p=0.29 respectively) and the relationship between lymphocyte nadir and OI was not significant (OR 0.09 p=0.053).
VZV/HSV and PJP ppx were prescribed to 42% and 54% respectively during treatment and continued for a median of 3 months post EOT (range 0-27, cessation date unknown in 60%). PJP ppx (sulfamethoxazole/trimethoprim) was associated with fewer bacterial infections (OR 0.44 p=0.003) but did not reduce the incidence of FN (OR 0.83 p=0.63). Antiviral ppx (aciclovir/valaciclovir) was associated with fewer VZV/HSV infections (OR 0.10 p=0.026). More ppx was prescribed in 2018-2019 (post GALLIUM) than 2011-2017 (PCP ppx - OR 5.19 p<0.001; VZV ppx - OR 3.76 p<0.001; Figure 2) with an associated fall in the number of infections per pt (IRR 0.55, p=0.011).
Factors independently associated with an increased number of infections (during and post EOT) were obinutuzumab vs rituximab (IRR 2.76, p<0.001), maintenance anti-CD20 (IRR 3.43 p<0.001), and stage III/IV disease (IRR 2.55, p=0.002). Factors specifically associated with infection post EOT were maintenance (OR 5.10 p<0.001) and obinutuzumab (OR 3.51 p=0.001). ECOG, hypogammaglobulinaemia, comorbidity index, treatment line and disease subtype were not associated with infections during or post treatment.
Conclusion:
iNHL pts receiving bendamustine are at high risk of prolonged lymphopenia and infectious complications extending beyond treatment completion, with half of infections occurring post treatment cessation. Lymphopenia duration and nadir did not correlate with infection. PJP and antiviral ppx reduced risk of bacterial and VZV/HSV infections respectively, though rates of PJP and VZV/HSV were low. Prolonged ppx to mitigate the risk of late infections should be considered, particularly in pts with additional risk factors such as concomitant obinutuzumab and anti-CD20 maintenance.
Manos:Bristol-Myers Squibb: Other: Travel. Di Ciaccio:Jansen: Honoraria, Other: travel and accomodation grant. Hamad:Abbvie: Honoraria; Novartis: Honoraria. Gregory:Janssen: Consultancy; F. Hoffmann-La Roche, Genentech, Inc., MSD, AbbVie, BeiGene, AstraZeneca, Celgene, BMS: Research Funding; F. Hoffmann-La Roche, Novartis, Sandoz, Gilead, AbbVie, MSD: Honoraria; F. Hoffmann-La Roche, Novartis, AbbVie: Speakers Bureau; F. Hoffmann-La Roche, Novartis, Sandoz, Gilead: Membership on an entity's Board of Directors or advisory committees. Gangatharan:Roche: Other: Travel grant. Hawkes:Merck Sharpe &Dohme: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Other: Travel, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding, Speakers Bureau; BMS celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck KgA: Research Funding; takeda: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal